A Critical Review of the Toxilogical Effects of Carrageenan
A Critical Review of the Toxicological Effects of Carrageenan and Processed Eucheuma Seaweed on the Gastrointestinal Tract
Samuel M. Cohen & Nobuyuki Ito
To cite this article: Samuel M. Cohen & Nobuyuki Ito (2002) A Critical Review of the Toxicological
Effects of Carrageenan and Processed Eucheuma Seaweed on the Gastrointestinal Tract, Critical
Reviews in Toxicology, 32:5, 413-444, DOI: 10.1080/20024091064282
To link to this article: https://doi.org/10.1080/20024091064282
Samuel M. Cohen
Department of Pathology/Microbiology, University of Nebraska Medical Center, 983135 Nebraska Medical
Center, Omaha, NE 68198-3135
Nobuyuki Ito
Nagoya City University Medical School, 1-Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467, Japan
ABSTRACT: Carrageenan is a high molecular weight, strongly anionic polymer derived from several species of red seaweed that is used for the textural stabilization of foods. Processed Eucheuma Seaweed (PES) is a form of carrageenan with a higher cellulose content. Food-grade carrageenan has a weight average molecular weight greater than 100,000 Da, with a low percentage of smaller fragments. Carrageenan is not degraded to any extent in the gastrointestinal tract and is not absorbed from it in species examined, such as rodents, dogs, and non-human primates. Systemically administered carrageenan has been reported to have a variety of effects, particularly on the immune system, but these are not pertinent to orally administered carrageenan. The substance poligeenan (formerly referred to as degraded carrageenan) is not a food additive. It exhibits toxicological properties at high doses that do not occur with the food additive carrageenan. In-long term bioassays, carrageenan has not been found to be carcinogenic, and there is no credible evidence supporting a carcinogenic effect or a tumor-promoting effect on the colon in rodents. Also, like many dietary fibers, there is significant cecal enlargement in rodents when it is administered at high doses, but this does not appear to be associated with any toxicological consequences to the rodent. Many toxicological studies on carrageenan have involved administration at doses in excess of today’s standards for dietary feeding levels in bioassays, and they are orders of magnitude in excess of those to which humans are exposed. Previous reviews of carrageenan and PES by the Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives (JECFA) have recommended a group allowable daily intake (ADI) of “not specified”. The lack of carcinogenic, genotoxic, or tumor-promoting activity with carrageenan strongly supports continuing such an ADI, and JECFA, during its most recent review in 2001, continued this recommendation. The various toxicological studies related to orally administered food-grade carrageenan are summarized along with a brief discussion of critical factors in intestinal carcinogenesis.
I. BACKGROUND INFORMATION ON CARRAGEENAN AND PROCESSED EUCHEUMA SEAWEED
Carrageenan is a high molecular weight (weight average molecular weight [Mw]>100,000 Da [Da]) sulfated polygalactan derived from several , species of red seaweeds of the class Rhodophyceae. It is used for textural stabilizationof foods and has no nutritive value (International Food Additives Council [IFAC] and Marinalg International, 1991). The most common forms of carrageenan are designated as kappa-, iota-, and lambda carrageenan (Norton and Goodall, 1983).
Kappa carrageenan is mostly the alternating polymer of D-galactose-4- sulfate and 3,6-anhydro-D-galactose. Iota carrageenan is similar but with the 3,6- anhydro-D-galactose being sulfated at the second carbon. Between kappa and iota carrageenan, there is a continuum of intermediate compositions that differ only in the degree of sulfation at the second carbon. Lambda carrageenan has alternating monomeric units composed mostly of D-galactose-2- sulfate (1,3- linked) and D-galactose-2,6-disulfate (1,4-linked). Carrageenan is the native extract of these galactose polysaccharides from specific seaweeds and is used by the food industry. In contrast is poligeenan. Poligeenan utililzes a different manufacturing process of seaweed that involves intentional extensive acid hydrolysis, resulting in sulfated galactose polymers with a weight average molecular weight of approximately 15,000 Da.
Poligeenan is used to a minor extent in the pharmaceutical industry (International Food Additives Council and Marinalg International, 1983; 1991; 1997; IARC, 1983; USAN, 1988). This distinction between carrageenan and poligeenan is essential because their physical and toxicological properties differ considerably. Comparing toxicological effects of poligeenan to the food additive carrageenan (Tobacman, 2001) is inappropriate. In addition, in contrast to carrageenan, poligeenan has no food performance properties, even at concentrations as high as 10% in aqueous food preparations, whereas carrageenan will function in food at concentrations as low as 0.01%. Consequently, poligeenan has no application as a food additive. Carrageenan contains little of the low molecular weight forms. The low molecularweight forms are <5% of the total composition of the commercial product (International Food Additives Council and Marinalg International, 1983; 1991; IARC, 1983, Uno et al., 2001). Because carrageenan is extracted from seaweeds under alkaline conditions, degradation to smaller polymerized units is avoided. As long as the pH is maintained above 6.0, carrageenan is stable to heat processing. Once carrageenan is in the gel configuration, as is the case becomes highly resistant to degradation, even under more acidic conditions (International Food Additives Council and Marinalg International, 1991, 1997).
Carrageenan ingested in the gel form is also stable to the conditions of passage through the digestive tract (Abraham et al., 1972; Benitz et al., 1973; Arakawa et al., 1988; Weiner, 1988). It has been reported that less than 6% of the excreted carrageenanis below 100,000 Da, and less than 1% is below 50,000 Da. Because of its large molecular weight, carrageenan remains within the lumen of the digestive tract and is not absorbed (Weiner, 1988, 1991).
Thus, there are no systemic effects of carrageenan following ingestion by rats, mice, or monkeys. In guinea pigs, it has been reported that carrageenan is absorbed to a slight extent (Engster and Abraham, 1976). This observation is based on the demonstration of staining of the macrophages of the lamina propria of the cecum and proximal colon by toluidine blue or by positive iron staining following administration in the drinking water of iron labeled carrageenan. However, even in guinea pigs, there is no evidence that the absorbed material is distributed systemically because there is no excretion in the urine. There is some evidence that carrageenan is detected in intestinal macrophages in guinea pigs (International Food Additives Council and Marinalg International, 1991, 1997). Generally, it can be concluded that orally administered carrageenan is not absorbed through the gut and is not metabolized to the lower molecular weight material (International Food Additives Council and Marinalg International, 1997).
Systemic administration of carrageenan has been shown to have various effects, particularly on the immune system (IARC, 1983; Weiner, 1991; JECFA, 1999). However, because carrageenan is not absorbed from the gas trointestinal tract, studies involving administration systemically are not relevant to a risk assessment of carrageenan exposure from foods or beverages.
PES, also known as semirefined carrageenan (SRC) or Philippine National Grade carrageenan (PNG), is derived from either E. cottonii (EC) or E. spinosum (ES). The predominant carrageenan type from EC is kappa carrageenan and from ES is iota carrageenan. PES is a semirefined form of carrageenan that utilizes a lower energy input in the refining process and produces a carrageenan with a higher percentage of cellulose and a lower inorganic salt content (Robbins, 1997). Few studies have been performed with PES. However, those that have been performed have produced results similar to those obtained with carrageenan. In the remainder of this article, carrageenan and/or PES is used where appropriate. However, because carrageenan and PES have virtually identical biological responses, where the word carrageenan appears in a summarizing or concluding statement the inclusion of PES is implied.
II. EFFECTS OF CARRAGEENAN AND PROCESSED EUCHEUMA SEAWEED
A. Long-Term Bioassays and the Evaluation of Carcinogenicity
Groups of five male and five female mice of two strains were administered 0, 0.1, 5, 15, and 25% carrageenan in their diet for their lifespan without evidence of adverse effects or carcinogenicity (Nilson and Wagner, 1959). Likewise, these investigators administered 0, 1, 5, 15, and 25% carrageenan in the diet for up to 24 months to groups of five male and five female rats of two strains without evidence of adverse effects or carcinogenicity except for the suggestion of hepatic sclerosis at the 25% dose level. Doses used in these mouse and rat studies were far in excess of today’s acceptable standard for oral dietary studies; the maximum limit is generally now considered to be no more than 5% of the diet (FDA, 1982; Grice, 1984; OECD, 1981, 1998).
Rustia, Shubik, and Patil (1980) conducted the most thorough long-term carcinogenicity study with rats and hamsters, utilizing kappa carrageenan derived from C. crispus. Seven week old MRC outbred rats and randomly bred Syrian golden hamsters from the Eppley colony were administered kappa carrageenan at doses of 0.5, 2.5, and 5% of the diet. Each dose group contained 30 females and 30 males of each species. Untreated controls received the same pelleted diet without added carrageenan, but there were 100 females and 100 males in each control group. These diets were administered for the lifetime of the animals, approximately 150 weeks for the rats and 110 weeks for the hamsters. There was no evidence of increased mortality, effect on weight gain, clinical signs of toxicity, or increased incidences of gross or microscopic lesions, including tumors. The only abnormality was an occasional soft stool consistency in some of the animals, particularly in the initial phase of the experiment. In particular, there was no increased incidence of erosion or ulcerations of the gastrointestinal tract mucosa. The average daily intake of carrageenan at the highest dose in the rats in this study was 4022 mg/kg/d and in hamsters it was 3719 mg/kg/d.In studies reported by Coulston (1975a, 1975b, 1976), two forms of carrageenan were evaluated in Sprague Dawley rats. RE-7063 was an extract from Hypnea that was a kappa carrageenan, and RE- 7064 was an extract from Iridaea, which was an extract between lambda and kappa carrag- eenan in its properties. Groups of 15 male and 15 female rats were administered either alpha cellulose as 5% of the diet, or one of the two forms of carrageenan at 1% or 5% of the diet for 1 year. There was a significant decrease in weight gain of the carrageenanfed rats compared with those given alpha cellulose. There were more Hematest positive stools in the alpha cellulose treated group than in the carrageenan-treated groups. Although there were occasional minor abnormalities in a few of the livers of rats treated with the carrageenan, there was no other evidence of abnormalities in the gastrointestinal tract, and there was no increase in tumor incidences in any tissues. The suppression of weight gain was well in excess of what would today be accepted as maximum tolerated dose, with growth retardation of 20 to 25%. There was no evidence of carrageenan like material observed in the liver by light or electron microscopy.
No longterm bioassays in rodents have been reported for PES (JECFA 1999). Based on a report by JECFA (1974), no deleterious effects were noted in the few dogs administered carrageenan during the course of pharmacological studies. No details were provided. In an experiment involving 40 Rhesus monkeys (19 males, 21 females), carrageenan was administered by gavage for the first 5 years of the experiment at doses of 0, 50, 200, and 500 mg/kg/ day, followed by administration at similar levels in the diet for an additional 2.5 years (Abraham et al., 1983). Stool consistency was decreased in a dose-related trend and positive fecal occult blood was observed. There was no difference in survival, and body weight changes were sporadic and not dose related. No gross or microscopic changes were seen, and no tumors were reported. In summary, chronic bioassays in rodents with carrageenan showed no evidence of carcinogenicity. Some of these experiments involved doses in excess of the current maximum standard of 5% of the diet and were even in excess of a maximum tolerated dose. Although experiments in dogs and monkeys were not sufficiently long to fully evaluate carcinogenicity, there was no evidence of preneoplastic changes in any tissue, including the gastrointestinal tract, in these species fed high doses of carrageenan.
A. Evaluation of Carrageenan in Bioassays Involving Intestinal Carcinogens
Carrageenan has also been evaluated in multistage models in rats, primarily evaluating its role as a possible colon tumor promoter. This involved administering carrageenan during and/ or after administration of a known colonic carcinogen, that is, subcutaneous injection of azoxymethane (AOM), subcutaneous injection of dimethylhydrazine (DMH), or intrarectal administration of N-methyl-N-nitrosourea (MNU). Table 1 summarizes the studies discussed in this section.
The first of these experiments was reported by Watanabe et al. (1978) and involved the administration of carrageenan (Viscarin 402) at a dietary concentration of 15%. Carrageenan was fed in the diet for 2 weeks prior to carcinogen treatment and continued for the entire course of the experiment. AOM (8 mg/kg/wk) was administered by subcutaneous injection for 10 weeks. In other carrageenan-treated groups, MNU was administered intrarectally, 2 mg per rat, 2 times per week for 3 weeks. In the AOM experiments, the rats were administered carrageenan for a total of 40 weeks and then terminated. In the MNU experiment, rats were fed carrageenan until they were terminated at 30 weeks. Groups of rats treated with AOM or MNU and fed control diet during the entire course of the experiment were included, as were groups fed carrageenan containing diet or control diet without carcinogen treatment.
Food intakes were similar between rats fed control diet compared with carrageenan containing diets (8.6 vs. 9.9 g/rat/day). However, there was a highly significant decrease of approximately 16% in weight gain between rats administered control diet compared with those administered the carrageenan diet (groups also treated with carcinogen had similar differences in weight gain). Rats fed the carrageenan diet showed edema and macrophage infiltration of the lamina propria and submucosa of the large intestine.
TABLE 1
Carrageenan Administered with Known Intestinal Carcinogens
TABLE 1 (continued)
A few rats showed a grayish white, coating like appearance of the mucosal surface in the distal part of the colon. Such changes in the rat intestine have not been observed by other investigators.
Except for an adenomatous polyp of the colon in one rat, tumors were only found in carrageenan treated rats that were also treated with AOM or MNU. Such polyps have not been observed in rats treated with carrageenan in other investigations.
In the AOM experiment, there were 26 rats administered AOM with carrageenan diet (Group 1),30 rats administered AOM with control diet (Group 2), or 15 rats in each group given the carrageenan diet only (Group 3), or control diet only (Group 4) without AOM. The incidence of colon tumors was 57, 100, 0, and 7%, respectively, with 1.5, 11.3, 0, and 1 tumors per tumor bearing rat in the four groups, respectively. In addition, two rats administered AOM with control diet (Group 1) had duodenal adenocarcinomas.
In the MNU experiment, there were 29 rats in the groups treated with MNU with control diet or with MNU with carrageenan diet. The incidence of tumors was 69 and 100%, with 1.5 and 4.4 tumors per tumor bearing rat, respectively.
The authors indicated that the diet that they used for their studies was marginal in its methionine content, and with the decreased body weight in the rats administered 15% carrageenan diet there may have been a deficiency of the methionine. In addition, the growth retardation clearly indicated that this dose of carrageenan was significantly in excess of the maximal tolerated dose (MTD). The authors also indicated that the carrageenan used in their study was without mutagenic activity in the Ames Salmonella assay, but specific data were not provided. In addition, the diet used in this experiment had a high fat content (20% corn oil).
Arakawa et al. (1986) evaluated a high dose of kappa carrageenan, 6% of the diet, administered in conjunction with subcutaneously injected DMH. The fat content of the diet was 2%. Rats were administered DMH + carrageenan (group 1), DMH only (group 2), carrageenan only (group 3), or control diet only (group 4), respectively. There were 20 rats in each of the first two groups and 15 rats in each of the last two groups. DMH was administered as weekly subcutaneous injections, 20 mg/kg for 16 weeks, and the carrageenan diet was administered for the entire 24 weeks of the experiment, both during and after carcinogen administration. Food intake among groups was similar, and the body weights were similar, although the carrageenan treated rats had approximately 10 g/rat lower body weight at the end of the experiment than the control diet fed rats. Rats given the carrageenan-containing diet had significantly greater fecal weight than did the control rats. The number of rats with tumors was 15/20, 8/20, 0/15, and 0/15 in Groups 1 to 4, respectively, with a total of 20 tumors in group 1 and 11 tumors in group 2. Interestingly, identical numbers of tumors were seen in the distal colon in groups 1 and 2 (8 distal colonic tumors in each), but there was an increased number of colonic tumors proximally in the DMH + carrageenan- treated rats compared to the DMH only-treated rats (12 vs. 3). Also, the number of larger tumors was greater in the DMH + carrageenan group. However, no diagnosis of adenoma vs. carcinoma was presented in this article.
Arakawa et al. (1986) also measured b-glucu-ronidase activity. In the colonic mucosa, liver and plasma, the level of b-glucuronidase activity was similar in carrageenan fed rats compared with control diet fed rats. However, the activity in fe- ces measured as activity per/mg of protein, was significantly lower in the carrageenan treated animals than in the control treated rats, most likely representing a dilutional effect secondary to increased fecal volume. The sialic acid content of the tumors and colonic mucosa did not differ between groups (Arakawa et al., 1989).
In a follow-up experiment, Arakawa et al. (1988) found the molecular weight distribution of the carrageenan in feces to be similar to that of the carrageenan that was fed in the diet, indicating that there was no degradation of the carrageenan in the gastrointestinal tract. Although there was an increase in the concentration and total amount of lithocholic acid in the feces in the carrageenan treated rats compared with the control rats, the concentration and total amount excreted was similar or decreased for cholic acid, chenodeoxycholic acid, deoxycholic acid, and hyodeoxycholic acid like substance. Importantly, the total bile acid concentration was significantly less in the carrageenan treated rats compared with controls, whereas the total amount of bile acids excreted was similar or slightly increased in the carrageenan treated rats compared with the controls. Lithocholic acid is known to enhance colonic carcinogenesis, but the decrease in the concentra- tion of most of the bile acids and slight increase in total amount of bile acids suggest that they are not responsible for the effects of feeding excessively high doses of carrageenan on DMH induced colon carcinogenesis.
Glauert and Bennink (1983) also found that total bile acids decreased following administration of a high dose (7.4% of diet) of carrageenan in a short term experiment (4 weeks). However, they did not find any evidence of increased epithelial cell proliferation in the colon of these animals.
The studies by Watanabe et al. (1978) and Arakawa et al. (1986) pose several difficulties for interpretation. They are clearly not promotion studies, but rather involve simultaneous administration of agents. In addition, both involve excessive doses of carrageenan, in excess of today’s standard maximum of 5.0% of the diet (FDA, 1982; Grice, 1984; OECD, 1981, 1998). One reason for this 5% limit is the likelihood of nutritional deficiencies and distortions compared with controls if a greater percentage of the diet is replaced. The experiment by Watanabe et al. (1978) involved a dose (15% of the total diet) in excess of the maximum tolerated dose that resulted in a decreased weight gain of approximately 16%. In addition to the overall toxicity in this experiment, nutritional effects, such as methionine deficiency and high fat content of the diet, might have contributed to the increased number of tumors.
Most often, effects resulting from the administration of an agent with a DNA-reactive carcinogen, such as DMH, AOM, or MNU, are due to an alteration of the metabolic activation or distribution of the carcinogen. This was likely the case in the experiments by Watanabe et al. (1978) and Arakawa et al. (1986) In both experiments, the excessive doses of carrageenan produced cecal enlargement and watery stools. This could well have altered the ability of the crypt cells to metabolize the carcinogen (DMH) or affected the handling of a highly reactive carcinogen such as AOM or MNU, either due to accentuated blood flow, altered bile acid concentrations, or other processes affecting carcinogen toxicokinetics in the intestinal tract. Generalized toxicity, decreased weight gain, and nutritional deficiencies could also alter the carcinogen toxicokinetics and metabolism.
In summary, the studies by Watanabe et al. (1978) and Arakawa et al. (1986) are not promotion studies, are not acceptable studies by today’s standards, and have numerous confounding factors, including dose greater than 5% of the diet, toxicity and nutritional deficiencies. It is inappropriate to consider them in an evaluation of the safety of carrageenan for human consumption.
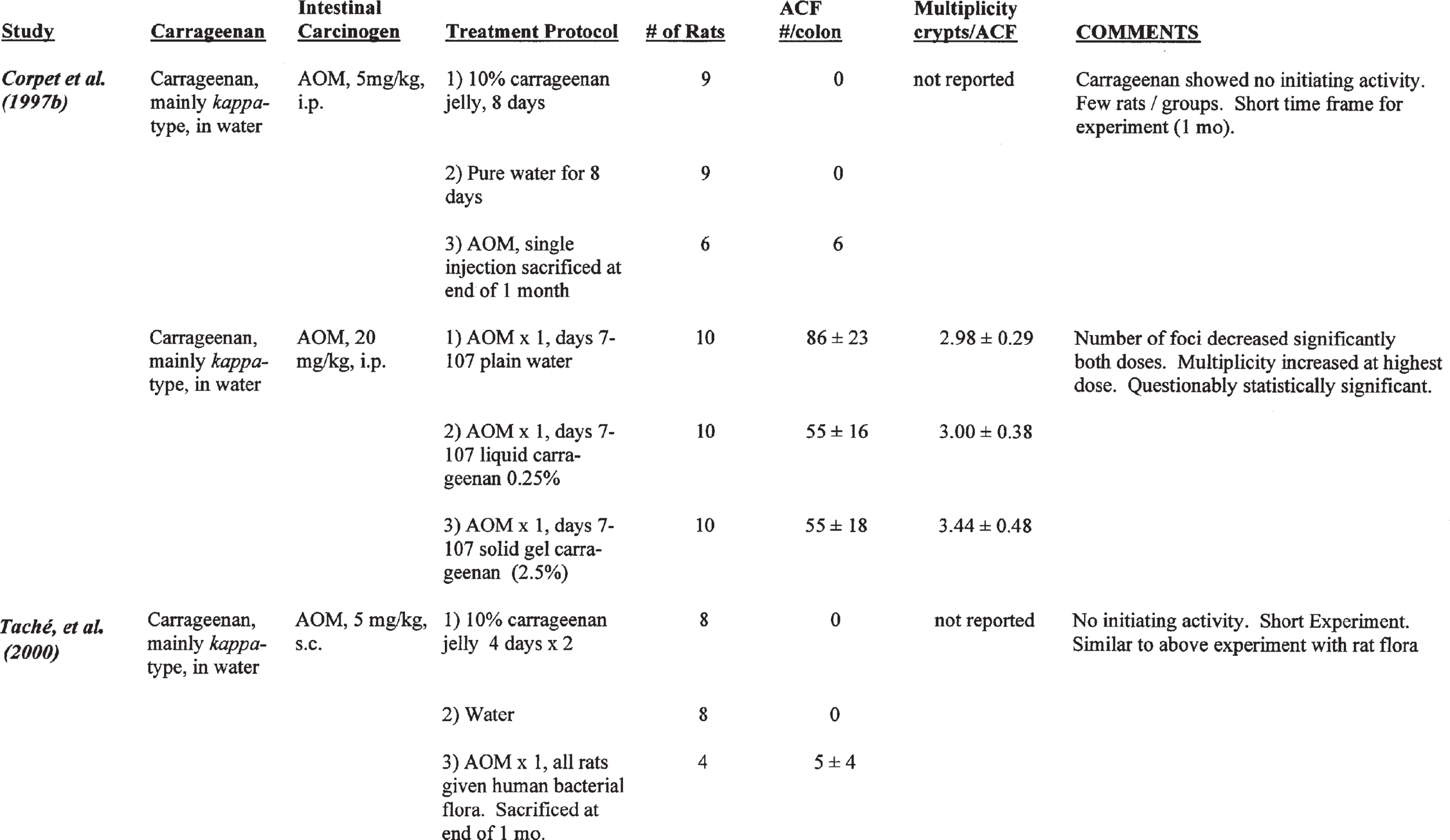
Corpet et al. (1997b) evaluated the initiating and promoting activity of carrageenan (mainly kappa form) in F344 female rats fed a low-fat rodent chow. To evaluate initiating activity, nine rats were administered jelly containing 10% carrageenan for 8 days (Group 1), 9 rats were given pure water for 8 days (Group 2) and six rats received an injection of AOM ip (5 mg/kg) as a positive control (Group 3). The rats were then sacrificed 1 month later. Aberrant crypt foci (ACF) were used as a surrogate marker for tumor formation. No ACF were found in either of Groups 1 or 2, and an average of 6 ACF/rat were found in the AOM-treated rats of Group 3. Thus, carrageenan showed no evidence of initiating activity.
Promoting activity was evaluated by the ad- ministration of AOM (20 mg/kg, ip), followed 7 days later with oral treatment with carrageenan in an aqueous medium. The rats received 0, 0.25% (liquid), or 2.5% (solid gel) carrageenan for 100 days. The results are shown in Table 2.
There was a significant decrease in the number of ACF per colon in rats orally treated with either 0.25 or 2.5% carrageenan in the water. However, there was a slight increase in the multiplicity of the lesions (crypts/ACF). No histologic description is given by these authors other than to state that no ulceration was detected. The diet used in this experiment was not stated. There was no effect on weight gain in the rats given 0.25% carrageenan, but there was a statistically decreased weight gain in the rats administered 2.5% carrageenan, although it was only about 5 to 6% different from controls. These data are difficult to interpret. On the one hand, there was a decrease in the number of ACF following oral administration of either 0.25 or 2.5% carrageenan.
TABLE 2
Results of First Promotion Experiment Reported by Corpet et al. (1997b) Administering carrageenan after AOM Initiation
In contrast, at the higher dose, 2.5%carrageenan, therewasaslightincreaseinthenumberofcrypts per focus, indicating that the size of the foci was increased but the number was actuallydecreased. Although the differences in number of crypts per focus was stated to be statistically significantly different from the AOM-control diet group, it is possible that this was within the variability that can occur with these foci, especially given the small number of rats per group. Wide variations in numbers and size of ACF have been observed by others in relation to several types of treatments, even AOM followed by control diet (Wargovich et al., 2000). In addition, although the raw data were not available for analysis, statistical evaluation based on means, sample size, and standard errors does not give statistical sig- nificance. Another issue is the contrast between the number of foci and the size of foci. If there is any effect at all, it is that carrageenan actually inhibited the number of foci. This would suggest that it has a chemopreventive rather than a promoting effect. Putting these results in terms of traditional initiation promotion experiments, one would have to actually classify carrageenan asan antipromoting substance rather than as a promoting or tumor enhancingsubstance.
An additional difficulty with this experiment is extrapolating results using ACF as a predictor of eventual carcinoma formation. Although there is substantial qualitative evidence linking ACF as a precursor lesion to colon carcinoma (Fenoglio- Preiser and Noffsinger, 1999), there is also substantial evidence that this relationship is not quantitative (Thorup et al., 1994, 1995; Thorup, 1997; Kristiansen et al., 1997; Kristiansen, 1996). It is clear that most ACF do not progress to carcinoma during the lifetime of the animal, and no morphologic, immunologic, or molecular marker has yet been identified that can predict which ones or what proportion will progress to adenoma or carcinoma. An initiation promotion protocol involving two agents complicates interpretation of these foci further. In addition, diet clearly influences the induction and progression of ACF (Thorup et al., 1992, 1994, 1995; Thorup, 1997; Kristiansen,
1996; Kristiansen et al., 1995, 1996, 1997).
Corpet and his colleagues (Taché et al., 2000) followed this initial study with further investigations evaluating the possible role of intestinal bacterial flora. Germ-free, female F344 rats were maintained under strict microbiological isolation in Trexler-type isolators. All materials used for the experiment, including cages, bedding, AOM, water, and carrageenan jelly, were sterilized either by filtration or steam heating, as appropriate for the substance. All the animals were administered human fecal flora prepared specifically for this study from healthy children. To evaluate the initiating activity of carrageenan, eight rats were administered jelly containing10% carrageenan for two 4-day periods. A second group of eight rats was given water (negative controls), and four rats were injected with 5 mg/kg AOM to serve as positive controls. This design mimicked the initiation study that they previously had reported in conventional rats. In these human flora associated (HFA) rats, no ACF were found in either of the first two groups given carrageenan containing jelly or water, but the positive controls (AOM group) had 5 ± 4 ACF. These studies show that in the HFA rats carrageenan had no initiating activity, similar to what was found in conventional rats.
To evaluate promotion, 20 mg/kg AOM was injected, followed 7 days later by oral administration of either water, 0.25% carrageenan (liquid), or 2.5% carrageenan jelly (solid). Each treatment group contained 10 rats. The second phase of the experiment lasted for 100 days, similar to the previous promotion experiment with conventional rats. However, in the experiment using HFA treated rats, there was no difference in ACF among groups. There was little difference in the molecular weight of the carrageenan administered and excreted by the HFA treated animals compared to conventional rats. However, there was slightly less carrageenan excreted in the feces of the HFA treated rats than in the conventional rats, suggesting that the dose given the conventional rats was equivalent to approximately 1.5 time that given the HFA rats. This corresponds to intake estimates of 2.5 g/kg for HFA rats compared with 4.0 g/kg for the conventional animals. This experiment indicates that if there is a promotion effect in the conventional rats from high oral doses of carrageenan, the effect is either lost completely in the presence of human bacterial flora or is lost due to a very slight decrease in the dose.
However, as indicated above, there is some doubt that there was an effect on the number of crypts per focus in the original experiment even though the difference was reported to be statistically significant. Such a conclusion is further supported by the results of an additional experiment performed by Corpet and his colleagues (Taché et al., 2000; Corpet, personal communication), utilizing conventional rats housed in isolators similar to those used in the HFA-treated rat experiment. This experiment utilized conventional rats administered either water or carrageenan as 2.0 or 2.5% of the water (both in gel form), with 10 animals/group. Again, AOM was injected in 20 mg/kg, followed 7 days later by carrageenan, which was administered for 100 days. The size of the ACF (ACF multiplicity, crypts/ACF) in HFA rats maintained in isolators with bedding was similar to the size of ACF seen in the experiment in which conventional rats were kept in isolators with bedding. In these experiments, there was no difference in the number of aberrant crypt foci among groups.
Overall, the studies by Corpet and his colleagues (Corpet et al., 1997b; Taché et al., 2000) clearly indicate that there is no initiating activity by carrageenan, even at a dose as high as 2.5% in water. However, there is a dubious suggestion of promoting activity in the colon. The reason for doubt for this experiment is that relatively small numbers of animals were utilized; a short experimental treatment period was utilized without tumors as an endpoint, the statistical significance is marginal in the presence of a highly variable parameter (number of crypts per foci), and there was actually an inhibitory effect on the number of ACF, although the size of the ACF was marginally increased at the highest dose. In addition, these investigators could neither repeat their results in a subsequent experiment utilizing conventional rats nor were the results reproducible when carrageenan was administered to human flora associated treated rats. The experiments were run at different times under different conditions, confounding the interpretation even further. In addition, chronic bioassays with carrageenan in rats showed no evidence of preneoplastic changes in the intestines, including the lack of a proliferative or adenomatous effect. Substances that have been demonstrated as promoters of colon carcinogenesis increase crypt cell proliferation when administered without prior treatment with a known colon carcinogen (Fenoglio-Preiser and Noffsinger, 1999).
In a recently completed study in Japan (Hagiwara et al., 2001), no promoting activity was observed in F344 male rats fed carrageenan at doses up to 5% of the diet for 32 weeks after pretreatment with DMH (20 mg/kg bw, s.c. weekly for 4 weeks). Colonic tumors rather than ACF were used as the end-point for this study. However, 0.2% cholic acid in the diet was also negative in this experiment, indicating that the positive control group did not respond.
Recently, Suzuki et al. (2000) reported that carrageenan in hibited gap junctional intracellular communication in rat liver epithelial cells in an invitro assay system. The results of this assay are difficult to interpret, because the effect was transient; a relatively mild effect was present at 15 min but returned to normal by one hour. Also, the system is in vitro and involves utilization of hepatocytes. As discussed above, carrageenan is not absorbed when ingested orally and would not be anticipated to have an effect on the liver. Also, this assay system is not specific for tumor promoters, being positive for a variety of other agents, including known anticarcinogens such as retinoic acid (Danz and Ur- ban, 1979; Zeilmaker and Yamasaki, 1986; Ruch and Klaunig, 1986; Klaunig and Ruch, 1990). Further more, it does not discriminate between chemicals that are positive in various animal systems, whether rats and/or mice, compared with the results in humans. For example, phenobarbitol, butylated hydroxyanisole (BHA), and sodium saccharin have been positive in this assay, but are known not to be carcinogenic in humans. Culture conditions can greatly affect the outcome of the studies, including effects on pH and cytotoxicity (Miller et al., 1987; Bohrman et al., 1988; Ruch et al., 1990). Suzuki et al. (2000) were also able to demonstrate that the effect at 15 min with l-carrageenan was not due to phosphorylation or localization of connexin 43, a critical gap junctional protein. The findings in this in vitro system do not always correlate with changes in vivo (Klaunig and Ruch, 1990; Holden et al., 1997). For example, phenobarbitol appears to have affects in vivo on gap junctional communication, but findings in vitro with bladder or skin epithelial cells are not always translated to positive effects in vivo.
In summary, the studies by Watanabe et al. (1978) and Arakawa et al. (1986) are not promotion studies and are seriously flawed methodologically. They are not appropriate for consideration for human safety. The studies by Corpet and his colleagues (Corpet et al., 1997b; Taché et al., 2000) do not show promoting activity, and the effects detected in their first study have not been reproducible. It is readily apparent from the above analysis that carrageenan does not have a carcinogenic effect in experimental animals, and it does not have a promoting effect for colon carcinogenesis in rats.
A. Evaluation of Genotoxicity
Carrageenan has been evaluated extensively for genotoxic activity in various in vitro and in vivo assays and has been uniformly negative, that is, nongenotoxic, in these assays. The various assays have been listed in the reviews submitted previously to JECFA (IFAC and Marinalg International, 1997) and are summarized in the following table (Table 3). These assays cover a wide variety of end points, including point mutational assays, such as those involving Salmonella typhimurium, with or without S9 activation, sister chromatid exchange assays, cytogenetic assays, dominant lethal assays in rats, as well as host mediated as says, mouse micronucleus assay, and the rec assay in Bacillus subitilis. The evidence strongly indicates that carrageenan is without genotoxic activity (IARC, 1983).
In addition to lacking genotoxic activity in a variety of in vitro and in vivo screens, based on its chemical structure it is highly unlikely that carrageenans would interact with DNA because carrageenan is a strongly anionic structure. Interaction with DNA requires formation of reactive cations or free radicals (Miller and Miller, 1977; Klopman and Rosenkranz, 1994).
PES from Eucheuma cottoni and Eucheuma spinosum have been evaluated in the Ames assay with and without activation with S9 mix and have shown no evidence of genotoxicity (Jackson, 1997; Sylianco et al., 1993). Although the Sylianco as says were considered to be inadequate in the 1999 JECFA review (JECFA, 1999), the review committee accepted the Jackson (1997) assays as being adequate and “considered that no further studies of genotoxicity were required”, as processed EC and ES were “not mutagenic in well conducted assays for reverse mutation in Salmonella typhimurium strains” (JECFA, 1999).
In summary, carrageenan and PES are regarded as nongenotoxic substances based on chemical structure and the results of a variety of in vitro and in vivo bioassays.
III. INVESTIGATIONS REGARDING THE INTESTINES
A. Cell Proliferation
Increased cell proliferation has been postulated as an important component of carcinogenesis (Cohen and Ellwein, 1990, 1991). However,
TABLE 3
Studies Evaluating the genotoxicity of Carrageenan and Processed Eucheuma Seaweed (Table Modified from IFAC and Marinalg International, 1997). Results of all Studies were Negative.
This is much too broad a statement because there are several aspects of increased cell proliferation that must be considered in determining a relationship to carcinogenesis. Cancer arises from multiple (at least two) genetic alterations, either inherited through the germ line or arising due to somatic mutations. In addition, although DNA replication is extraordinarily accurate, rare (approximately one mistake per 1010 nucleotides per replication) mistakes occur. Thus, an agent can increase the risk of cancer by either directly damaging DNA (DNA adduct formation) or by increasing the number of DNA replications or both. A critically important determinant is whether the increased proliferation is occurring in the stem (pluripotential) cell population of the target tissue. Also, it is the number of DNA replications that is the critical parameter, not the rate. The number of replications can be increased either by increasing the rate or increasing the number of stem cells. Frequently, increased number and rate both occur, but not always. In addition, DNA replications can be increased by increasing cell births, either by direct mitogenesis or by toxicity and regeneration, or by decreasing cell deaths (which increase cell number) by inhibiting apoptosis or differentiation.
Included in the stem cell population of the colon are the few cells at the base of each crypt that has pluripotential capabilities, that is, they are the cells responsible for the regeneration of the tissue if it is injured (Wright and Alison, 1984; Bykorez and Ivaschenko, 1984; Bach et al., 2000). These cells gradually migrate up the length of the crypt and almost immediately become committed to differentiation. Terminal differentiation into mucinproducing cells, the goblet cells, ultimately occurs at the tips of the gland. It is only the proliferating stem cells at the base of the crypt that are the cells that ultimately evolve into tumors in the process of carcinogenesis. These can undergo carcinogenesis either as flat, dysplastic lesions, as seen in hereditary nonpolyposis colon cancer (the two Lynch syndromes), or, much more commonly, expand into tubular and/or villous adenomatous polyps that in a minority of instances can evolve into carcinomas. The mouse and rat models of colon carcinogenesis generally follow the polyp sequence. Adenomatous polyps, whether villus or tubular, are a proliferation and an accumulation of the stem cells of the crypt.
In contrast, hyperplastic polyps are an accumulation and increased proliferation of the cells committed to differentiation and the fully differentiated mucinproducing goblet cells (Owen and Kelley, 1996; Fenoglio Preiser, 1999). Because these cells are not stem cells, these polyps do not evolve into carcinomas. Hyperplastic polyps, even in patients with literally thousands of such polyps, do not pose an increased risk of developing into carcinoma of the intestine. Hyperplastic polyps are extraordinarily common, with most humans that live beyond the age of 70 ultimately having one or more of these lesions. However, because they are a proliferation of nonstem cells, they are not preneoplastic. Thus, the simple statement of increased cell proliferation leading to increased risk of cancer is not accurate. There must be an increased proliferation of the stem cell population, and in the instance of the colon that represents the cells initially at the base of the crypts of the intestinal glands.
The colonic mucosa is a highly complex tissue composed of a variety of cells (Levine and Haggitt, 1992; Owen and Kelly, 1996). In addition to the epithelium, which is basically a single cell layered structure with the three basic cell types, that is, stem cells at the base of the crypt, differentiating cells along the walls of the crypt, and fully differentiated, mucinproducing goblet cells at the tips of the glands, there are also a wide variety of cell types in the lamina propria. These include the endothelial cells lining the blood and lymphatic vessels, occasional fibroblasts, and a large proportion of mononuclear inflammatory cells, predominantly lymphocytes and macroph- ages, but also including plasma cells, eosinophils, and mast cells. In addition, the number of these mononuclear cells, particularly lymphocytes and macrophages, can be increased under normal circumstances, even forming lymphoid aggregates, the equivalent of Peyer’s patches that are seen in the small intestinal mucosa. The extent of mono- nuclear infiltration of the lamina propria can be influenced by a variety of factors, including type of diet, timing of diet to the time of biopsy or sampling, level of hydration, bacterial microflora, level of fiber, fat, or protein in the diet, as well as numerous other components of feces, including transit time in the colon. Because of the complexity of the cellular composition of the colonic mucosa, assessment of the relationship of cell proliferation to colon carcinogenesis tends to be somewhat more difficult than in some other tissues. Essential to the evaluation of colonic proliferation and its relationship to carcinogenesis, is careful assessment of the proper cell type, that is, the stem cell population of the crypts. The critical determinant in assessing cell proliferation as it relates to carcinogenesis is the number of pluripotential (stem) cells that are proliferating. Thus, an appropriate assay must use as the denominator only the number of stem cells, not other cell types. This can only be accomplished using morphologic methods such as autoradiog- raphy for 3H-thymidine incorporation or immunohistochemistry for bromodeoxyuridine (BrdU) incorporation into DNA or staining for endogenous indicators of DNA replication such as proliferating cell nuclear antigen (PCNA). Using such morphologic methods, the number of DNA synthesizing (labeled) stem cells can be counted to determine the numerator and the total number (labeled and nonlabeled) of stem cells as the denominator. Stem cells can be specifically identified and counted. The biochemical methods using mucosal cell homogenates to assess 3H-thymidine or BrdU incorporation or the thymidine kinase activity cannot distinguish cell types (Troncale et al., 1971; Wright and Alison, 1984). Thus, these biochemical methods are not appropriate assays to evaluate cell proliferation related to carcinogenesis in the gastrointestinal tract.
Originally, the only method available to accurately make morphologic assessments was the mitotic index. This is an extremely laborious method, and because of the relatively short time of the cell cycle in which the cells are in mitosis it is not very sensitive (Wright and Alison, 1984). A better marker was developed with the ad- vent of 3H-thymidine and autoradiographic morphologic evaluation (Wright and Alison, 1984). 3H-thymidine is incorporated into the DNA during S phase. The major benefit of this method was its increased sensitivity because of the longer time that cells are in S phase and thus the larger number of cells being labeled with this marker of cell division. In addition, it is a relatively easy method and allows for easy identification and counting of specific cell types, such as stem cells in the crypts. This avoids counting all cells, which may be quite misleading. Crypt cells normally account for only a very small fraction of the cells in the colonic mucosa. Labeling with the nonradioactive marker bromodeoxyuridine (BrdU) using immunohistochemical staining has essentially replaced 3H- thymidine methods because it has similar sensitivity and precision and avoids the use of a radioisotope (Eldridge et al., 1990). Specific cell types can be assessed. Other morphologic mark- ers have been identified in recent years utilizing immunohistochemical techniques, with the markers being associated in one way or another with various stages of the cell cycle. These include such markers as proliferating cell nuclear antigen (PCNA) (Dietrich, 1993), Ki-67 (MIB-1) antigen (Perret et al., 1997) and Ki-S2 antigen (Rudolph et al., 1998). The advantages of these latter mark- ers are that they are endogenous to the tissue and do not require administration of an exogenous agent such at 3H-thymidine or bromodeoxyuridine. However, they are not as specific for the S phase of the cell cycle and can give some misleading results under certain circumstances (Dietrich, 1993).
In addition to the need for counting specific cells, rather than just the amount of activity in the tissue as a whole, one needs to take into account the various factors that can alter the labeling index that is utilized. For example, diet is a major factor (Glauert and Bennink, 1983; Caderni et al., 1989; Hambly et al., 1997; Lee et al., 1993). Specifically, cholic acid has been suggested to increase colonic cellular proliferation (Cohen et al., 1980). Another critical factor is the relationship of the time the animals are sacrificed to the time they last ate. There is a marked diurnal variation in all of the labeling indices that have been examined, with greater than a twofold difference at different times of the day or night, dependent on the relationship to the time the animals have been eating and drinking (Sigdestad et al., 1969; Wright and Alison, 1984). Fasting of animals prior to sacrifice for determination of a labeling index, a method used frequently by some investi- gators, unfortunately can markedly alter the interpretations and conclusions of a study, because there is a response of the tissue to the condition that the animals are fasted. This may be totally unrelated to an effect of the treatment on cell proliferation, but may be a response of the animal to the withdrawal of the treatment. This can happen extremely rapidly, within a matter of hours. There have been few studies in which carra- geenan has been evaluated for its effect on cell proliferation in the colon, and the results are pre-sented in Table 4.
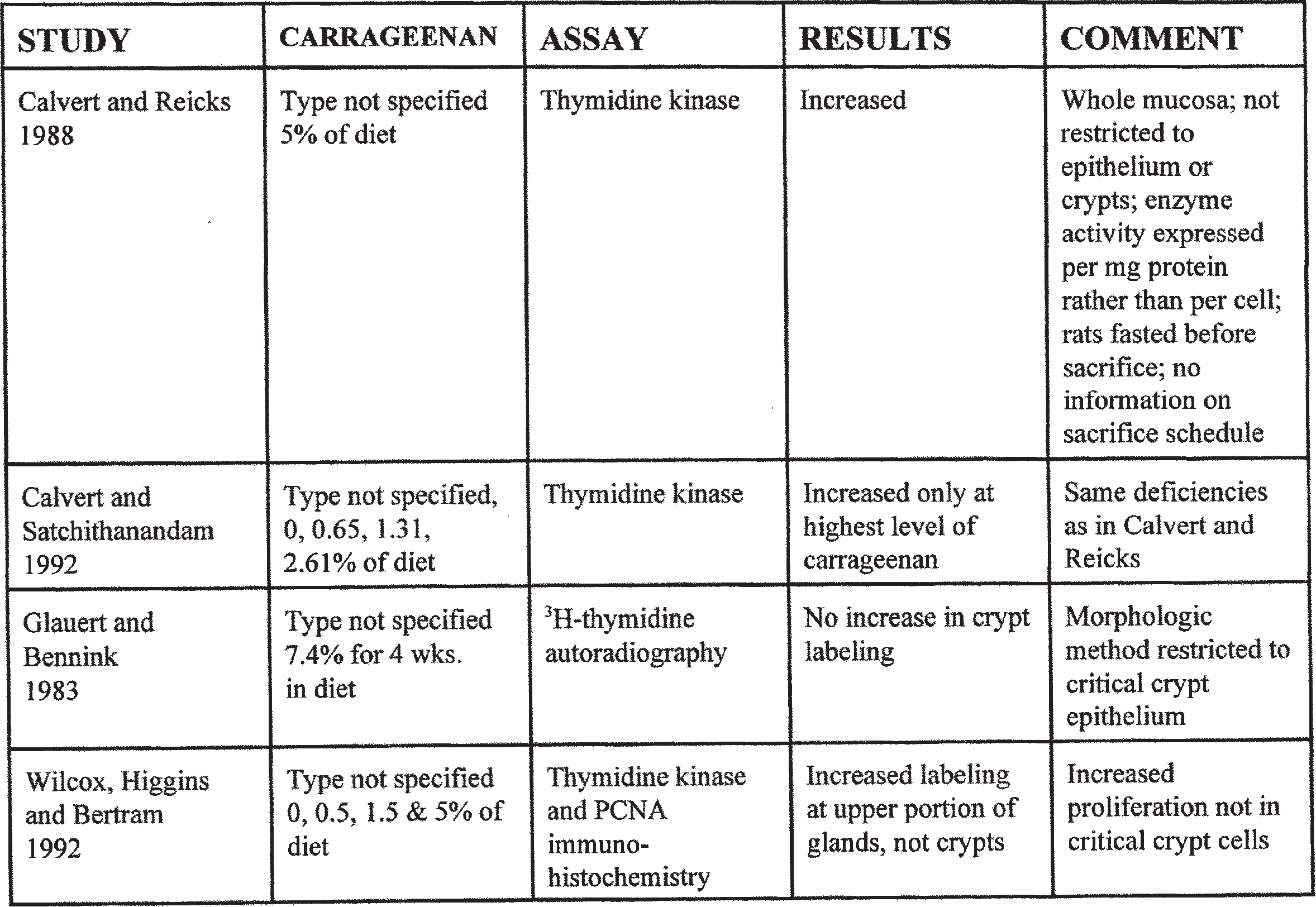
Calvert and Reicks (1988) utilized thymidine kinase enzyme activity as their assay system for assessing cell proliferation of the colon. Thymidine kinase is an important enzyme in DNA synthesis, and its activity correlates with the S phase of the cell cycle. However, the methodology used by Calvert and Reicks (1988) does not take into account any of the issues discussed above. The method involves scraping of the mucosa, digestion of the cells and an assessment of the thymidine kinase activity relative to the amount of protein in the tissue. For assessment of colon proliferation, there are several difficulties with this methodology.
Because the entire mucosal tissue is mixed together, including all cell types, there is no way to specifically assess proliferation in the critical stem cells. As the crypt stem cells represent a very small percentage of the total number of cells in the mucosa, on increase in proliferation in these cells would not be detectable with a method such as thymidine kinase activity, which represents the activity in all cells that are present.
In addition, thymidine kinase activity was expressed per unit of total protein rather than by the number of cells. Total protein is not an ad- equate surrogate marker for cell number because the amount of protein in the cells can vary enormously, particularly if there is hypertrophy of the cells as is frequently seen with cecal enlargement. This could also vary markedly among tissue samples depending on the mucin that is present compared with the amount of other cellular components such as collagen, or due to the level of edema, albumin, or even red cells that are present in the blood vessels in the tissues (Levine and Haggitt, 1992). In the report by Calvert and Satchithanandam (1992), they mention that there was vascular congestion and erythema of the distal 4 to 5 cm of the colon after carrageenan administration. The only denominator that is acceptable to use to evaluate cell proliferation with respect to carcinogenesis are the specific targeted cells, the crypt stem cells of the colonic crypt.
TABLE 4
Effect of Carrageenan on Cell Proliferation in the Intestine
In addition, the vascular congestion, erythema, and edema will alter the protein content without pro- portionally increasing cell number, making it impossible for Calvert and Satchithanandam (1992) to determine cell number.
In addition to these significant difficulties in interpreting the response, the animals in the Calvert and Reicks (1988) study were fasted before sacrifice for determination of the thymidine kinase enzyme activity. Also, there was no information in the report as to whether the animals were all killed at the same time of day, at a similar time of day, or whether the sacrifice of the animals was spread out over several hours.
In a further experiment, Calvert and Satchithanandam (1992) reported the results of an experiment comparing different doses of carrageenan on cell proliferation utilizing the same thymidine kinase activity assay as used in the previous experiment. The doses evaluated were 0, 0.65, 1.31, and 2.61% carrageenan in the diet. As in the previous experiment, the animals were fasted overnight before sacrifice. There were no histologic abnormalities associated with the carrageenan feeding, and there was only a significant increase in thymidine kinase activity in the animals administered the highest dose of carrageenan. This experiment has the same deficiencies and difficulties in interpretation as the first experiment and cannot be used to evaluate the effect of carrageenan on proliferation of the crypt stem cells. In both of these experiments, the entire colon was evaluated, which also poses difficulties in interpretation since the response to many agents may be limited to one part of the colon. Glauert and Bennink (1983) performed anexperiment in which rats were fed diets containing 7.4% carrageenan. Cell proliferation in the colon was evaluated utilizing a morphologic, 3H- thymidine, autoradiographic methodology that can specifically evaluate the colonic crypts. There was no significant increase in cell proliferation in the crypts, providing strong evidence that the increase in thymidine kinase activity seen by Calvert and his associates (Calvert and Reicks, 1988; Calvert and Satchithanandam, 1992) in the two experiments described above was not related to an increase in proliferation of the stem cells of the crypt but represented increased activity due possibly to proliferation of other cell types in the colon. As described in the section on cecal enlargement, the increase in thymidine kinase activity was most likely related to an increase in proliferation of the committed and differentiated cells of the intestinal glands, as well as possibly an increase in the proliferation of the nonepithelial cells in the lamina propria. In addition, because of the use of protein as the denominator in the studies by Calvert and colleagues (Calvert and Reicks, 1988; Calvert and Satchithanandam, 1992) rather than cell number, it was likely that an artifact introduced due to the hypertrophy of the epithelial cells frequently associated with cecal enlargement.
If there is any thinning of the mucosa, as frequently occurs when there is increased fecal production, as is seen in response to carrageenan administration at high doses, there is no way to assess how much variability there is in the nonepithelial component of the material that is being evaluated unless there is direct morphologic microscopic evaluation of the tissue. The controls would more likely have less thinning of the mucosa and the proportion of epithelial cells compared with nonepithelial cells could be dramatically different between these samples and thus lead to marked differences in activity. In addition, as mentioned above, there is no way to assess the proliferation of the critical cell type, the stem cells of the crypts. Even minor variations in the amount of mononuclear infiltrate among animals could give grossly different results, which would be misleading with respect to interpretation of crypt cell proliferation. Nonmorphologic methods are fraught with these dangers.
In the studies by Arakawa et al. (1986), an effect by carrageenan on tumor number was seen only in the proximal colon, not the distal colon. The difficulty in interpreting these experiments involves all of the above difficulties, because they utilized total protein as their denominator, utilized thymidine kinase activity as their marker for cell proliferation, utilized mucosal scrapings of the entire colon rather than specific parts of the colon, and took their samples after a period of fasting of the rats prior to sacrifice. In addition to all of these difficulties, the method, as indicated above, does not allow for specific evaluation of the cell type critical to carcinogenesis. In fact, it was likely not to have been evaluated then, because the crypt stem cells represent a very small proportion of the total number of cells that would be taken in a total mucosal scraping, likely accounting for less than 1% of the total cells. It is more likely that the increase in thymidine kinase activity reflected an increase in some other cell types.
In a study reported by Wilcox et al. (1992), increased thymidine kinase activity was observed following carrageenan administration at the 5% level of the diet but not at 0.5% or 1.5% of the diet. However, utilizing PCNA and BrdU labeling, they were able to demonstrate that the increase in labeled cells in the epithelium was restricted to the upper portion of the glands, not the base of the crypt where the stem cells are located. This supports the hypothesis that the proliferation occurring in response to high doses of carrageenan in the diet are occurring in cells that are unrelated to the carcinogenic process, whether in the epithelium or in nonepithelial tissues. The observation that the increased proliferation in the epithelium is localized to the upper portion of the gland rather than the stem cells in the lower portion of the crypt correlates well with observations from other substances that produce cecal enlargement, such as antibiotics and certain sodium salts, which produce an increase in the number of mucinproducing, goblet cells in the colonic glands rather than a proliferation of the stem cells of the crypts (Wilcox et al., 1992).
In summary, there are only four experiments that have evaluated the effects of carrageenan on intestinal cell proliferation. The one by Glauert and Bennink (1983) utilized a morphologic method and showed no increased proliferation. Wilcox et al. (1992) observed an increase in thymidine kinase activity, but using morphologic methods found the increase in proliferation in the upper portions of the gland rather than the crypts. The experiments by Calvert and associates (Calvert and Reick, 1988; Calvert and Satchithanandam, 1992) utilized a methodology that is uninterpretable with respect to colon carcinogenesis and, in fact, was more likely observing an increased proliferation of a cell type that is unrelated to the carcinogenic process. Thus, it is highly unlikely that carrageenan is increasing cell proliferation of the critical crypt stem cells that are necessary for colonic carcinogenesis.
A. Cecal Enlargement in Rats
A generally consistent observation in rats administered high doses of carrageenan is the presence of cecal enlargement (Leegwater et al., 1974). This is usually accompanied by soft, watery stools, and even obvious diarrhea. Cecal enlargement in rodents in general, and especially in rats, is a common response following administration of an incompletely absorbed carbohydrate such as carrageenan or a variety of other fibers (Newberne et al., 1988). Cecal enlargement is generally produced by many plant derived and related hydrocolloids, including not only carrageenan but also by other fibers such as carboxymethylcellulose, guar gum, gum acacia, locust bean gum, pectin, oat bran, and wheat bran (Elsenhaus et al., 1981; Mallett et al., 1984, 1985). Cecal enlargement is also seen following administration of any substance that leads to abnormalities in water absorption from the cecum. Such treatments include administration of high doses of various chemicals, such as sodium saccharin (Anderson, 1983), sodium ascorbate, (Cohen et al., 1991), and a variety of others. It is also seen commonly in animals treated with relatively high doses of antibiotics (Bertram, 1991). This latter response has been presumed to be related to an alteration in gastrointestinal bacterial flora in response to the antibiotic treatment.
Cecal enlargement is a very common responseto a variety of treatments in rodents. As just mentioned, this is particularly characteristic of rats, but also occurs commonly, although usually to a lesser extent, in mice, hamsters, and other rodents. The response of cecal enlargement has also been observed in other species, such as dogs, but to a considerably lesser degree (Bertram, 1991). Cecal enlargement has not been reported as a response to treatment with high concentrations of dietary fiber in primates, whether non-human or human (Bertram, 1991).
The enormous cecal enlargement that occurs in rats in response to a variety of treatments complicates to some degree the interpretation of the effects of these treatments and makes extrapolation of the results to humans difficult. Adding to the complexity of this interpretation is the fact that cecal enlargement involves certain alterations in cellular kinetics that can modify other toxicologic endpoints, such as increased cell proliferation and even carcinogenesis.
As indicated, cecal enlargement is accompanied by increased water content of the stools frequently and is accompanied by diarrhea (Newberne et al., 1988). A number of alterations occur, especially with cecal enlargement, that are associated with the increased fecal water content. These changes include hypertrophy of the mucosal epithelial cells, as well as an increase in cell number. There is also an indication that the level of cell proliferation in the cecum as a whole increases (Newberne et al., 1988). However, specific cell types that are proliferating have not been identified. Based on histopathologic evaluation of the cecum associated with enlargement, the increase in cell number occurs in two major populations (Newberne et al., 1988; Bertram, 1991).
1. There is an increase in the number of mono- nuclear cells, particularly lymphocytes and macrophages in the lamina propria, including an increase in the number and size of lymphoid aggregates.
2. There is an increase in mucus production and mucuscontaining cells.
Thus, it would appear that the increase in cell size and cell number are not related to the crypt stem cells, but rather are related to an increase in the mononuclear cells in the lamina propria and submucosa, as well as an increase in the differentiated, mucuscontaining cells of the epithelium. These are the types of changes that correspond to hyperplastic polyps in humans, although the mor- phology of the cecal mucosa associated with rodent cecal enlargement does not have all of the features of hyperplastic polyps. However, the implication of these changes is that even if cecal enlargement is associated with hypertrophy and hyperplasia, it is not associated with an increase in cell proliferation of the critical crypt stem cells that are important for carcinogenesis. Further evidence that these phenomena are not associated with carcinogenesis is the fact that they are completely reversible even if they have been present.
C. Effects on Microflora
In addition to cecal enlargement and watery diarrhea, high doses of carrageenan in the diet have a significant influence on the bacterial microflora of the cecum and the remainder of the colon of rats (Mallett et al., 1985). Because of the increase in fecal volume, largely due to increased water, the concentration of bacteria overall on a per gram basis is decreased in the cecal contents. This decrease in the concentration of bacteria is associated with a decrease in corresponding backterial related enzyme concentrations, such as azoreductase, b-glucosidase, b-glucuronidase, nitrate reductase, and nitroreductase (Mallett et al., 1985). Because the total volume of the feces is increased, the total number of bacteria and the total number of these enzymes is generally similar to controls. Like cecal enlargement, the effect of carrageenan on microflora is greatest in rats, affecting mice and hamsters to a lesser degree (Mallett et al., 1985). Also, in hamsters some of the enzymes actually are increased in concentration (Mallett et al., 1985). Carrageenan has no effect on bacterial viability itself (Mallett et al., 1985).
Different fibers have varying degrees of effect on the microflora, dependent on the potential for degradation and fermentation of the fiber by the microflora (Mallett et al., 1984; Michel and Macfarlane, 1996). This is largely dependent on the composition of the sugar backbone of the polysaccharide. Agar, which is also a sulfated galactosecontaining polysaccharide but with lower sulfate content, has effects on the microflora and associated enzymes similar to carrageenan (Mallett et al., 1984; Michel and Macfarlane, 1996). Agar also has the same effect on cecal enlargement and production of watery diarrhea when administered at equally high doses as carrageenan. Other fibers that have similar effects on the microflora of the rat include gum acacia and locust bean gum. In contrast, carboxymethylcellulose, guar gum, and pectin lead to an increase in the number of bacteria in the feces. The differences in response to these various fibers is largely related to the potential for fermentation, as well as the osmotic effects of the polysaccharide on the fecal composition.
Alterations in fecal composition can significantly influence the response to administration of a variety of chemicals, including dietary fibers (Rowland and Mallett, 1986). The response to different fibers is also greatly influenced by the overall composition of the diet. The response is influenced not only by the amount of fiber that is administered, but also by the amount of fat, other carbohydrates, and the mineral and salt cationic and anionic composition of the diet as a whole (Rowland and Mallett, 1986).
Changes in fecal composition, especially overall fecal bulk, can lead to an overall reduction in the concentration of administered chemicals, whether toxins, dietary components, or carcinogens (Corpet and Parnaud, 1999). Increased fecal bulk usually leads to a decreased transit time, thus the induction of diarrhea. Altering the composition of the feces also can lead to variations in the binding and inactivation of various chemicals in the fecal stream. This has been seen, for example, with the various cholic acids present in feces.
Dietary fibers can also potentially alter the toxic effects of various administered chemicals indirectly by diluting the overall concentration of the bacterial population that might then affect the chemical’s metabolism (Rowland and Mallett, 1986). This could also lead to variations in concentrations, metabolism, and absorption of a variety of dietary nutrients. Alterations in the intestinal epithelium and underlying tissues also can occur by alterations in the composition of the feces, particularly alterations leading to increased bulk, watery stools, and the presence of indigestible material such as the polysaccharide carrageenan. However, most of these effects occur only at relatively high doses of the administered fiber, because there is a lack of bulking effect on the feces at lower doses.
It has become apparent from numerous investigations that the effects of carrageenan, as well as other fibers are highly variable among species and strains, and dietary differences can have an enormous impact on the effects of these fibers on the gastrointestinal tract (Mallett et al., 1984; Rowland and Mallett, 1986; Salyers et al., 1977). This makes results from animal models, particularly at high doses, difficult to extrapolate to the human situation. It is essential to be able to take into account both interspecies differences as well as differences in dose.
IV. THEORETICAL FRAMEWORK FOR EVALUATING CARCINOGENESIS
A. Multistage Carcinogenesis
It has become apparent over the last several decades that cancer arises as a consequence of more than one genetic alteration occurring in a cell. The first model of multistage carcinogenesis actually was developed and presented prior to the elucidation of the structure of DNA and its role in genetics. The first multistage model was reported by Berenblum and Shubik (1947) in a landmark publication. This model has formed the basis for a considerable amount of research in ensuing decades, particularly related to chemical carcinogenesis. However, the terms initiation and promotion, and initiator and promoter, have been misused by numerous investigators, with little relevance to their original intent and description. Briefly, the overall properties of initiation and promotion were defined by Berenblum and Shubik and later extended by numerous investigators such as Boutwell, Slaga, Hecker, and Lutz, as well as numerous others, utilizing a variety of tissue models (Slaga, 1984). The original model described by Berenblum and Shubik (1947) utilized mouse skin as a model system. However, it is applicable to other types of tissues and tumors.
General properties of initiation and promotion are illustrated in Figure 1.
Initiation was defined as a single, small dose of a carcinogen that by itself did not lead to the production of tumors within the sample size and within the given time frame of the experiment (Group 1). The promoter was a substance such as croton oil used by Berenblum and Shubik (1947) that could be administered multiple times, producing a hyperplastic tissue response, but no tumors were produced in the span of time of the experiment (Group 2). The active ingredient of croton oil is now known to be the phorbol esters, such as tetradecanoyl phorbol acetate (TPA) (Slaga, 1984). If a single dose of initiator is followed by multiple doses of the promoter, tumors are produced (Group 3). Two features of the model were critical in defining the characteristics of initiator and promoter. First, if the application of the promoter began several weeks after the initiator had been applied (Group 4), the same incidence of tumors were produced as when the promoter was administered beginning immediately after the initiator (Group 3). Thus, it was clear that the initiating event has been embedded into the memory of the initiated cell. Today we realize that this means that an irreversible genetic event had occurred that can be passed on to daughter cells over time. The second key feature was that if the promoter was administered first followed by a single dose of initiator (Group 5) no tumors were produced in the time span of the experiment. Thus, the sequence of events in initiation and promotion are critical.
Later experiments (Boutwell, 1964) demonstrated that promotion was reversible until the lesions became malignant. This switch to a malignant state is due to an additional irreversible, most likely genetic, event resulting in conversion of the benign lesion to a malignant neoplasm. This step has been referred to as progression (Boutwell, 1964; Shubik, 1984; Pitot and Dragan, 1991).
FIGURE 1. Standard protocol illustrating basic features of initiation and promotion.
This model system has been utilized in nu- merous other organ systems in mice and rats in the last 5 decades, but with variations on this theme that make it difficult now to be able to precisely define initiator or promoter. Unfortunately, many investigators have used the terms simply to indicate that one chemical is administered first and then is followed by another. This clearly is not what is intended by the terms initia- tor and promoter, which imply several specific features related to sequence, duration, reversibility, and others. In addition, some investigators, such as in the reports by Watanabe et al. (1978) utilizing AOM or MNU and carrageenan, and by Arakawa et al. (1986), utilizing DMH and carra- geenan, administer both chemicals concurrently then continue to administer carrageenan until the end of the experiment. Superficially, this looks like administration of one chemical followed by another, but in reality represents a coadministration of two chemicals rather than initiation and promotion.
Although the model of initiation promotion progression has proven valuable in furthering our understanding of the multistage nature of carcinogenesis, it is clearly not a perfect model that can be utilized for all of cancer research (Cohen and Ellwein, 1991; Cohen, 1998). Nevertheless, it has been especially critical in identifying two classes of agents that act very differently in biological terms in the carcinogenic process. It is clear that certain agents directly affect the DNA, causing irreversible genetic damage. In some model systems, these agents are referred to as genotoxic (DNA-reactive) carcinogens. This term has been used synonymously with the term initiator, but it clearly must be distinguished from it. In contrast, promoting substances are not thought to cause irreversible DNA damage and are commonly grouped together by many under the term nongenotoxic carcinogens (Cohen and Ellwein, 1991).
In evaluating the carcinogenic activity of a given agent, a distinction between genotoxicity and nongenotoxicity is critical in the determination of its behavior as an initiating type of agent compared with a promoting type of agent. It is important that these terms be utilized properly so that misinterpretation and inappropriate conclusions are not drawn.
As indicated above, the experiments by Watanabe et al. (1978) and Arakawa et al. (1986) are not truly initiating promoting experiments. In addition, as described above, the studies are unacceptable and cannot be used in interpretation of the effects of carrageenan on colon carcinogenic or promoting activities.
The experiments by Corpet and his colleagues (Corpet et al., 1997b; Taché et al., 2000) are also difficult to interpret in terms of the initiation promotion model. In the experiments by Corpet and his colleagues (Corpet et al., 1997b; Taché et al., 2000), there was actually a decrease in the number of aberrant crypt foci (ACF) produced in rats given carrageenan after AOM. The incidence of animals with ACF appeared to be virtually 100% in all treatment groups, although the data are not presented. This clearly is not what is meant in the usual initiation promotion experiments, where the application of the initiator alone should result in either no tumors (lesions) or at most a few tumors (lesions). The incidence of rats with ACF was virtually 100% with the treatment with AOM alone. Treatment with 0.25% and 2.5% carrageenan after AOM also had an incidence of 100%, but led to a decrease in the number of ACF in the treated animals. This is contrary to what would be expected from the definition of the initiation promotion model. The only effect from carrageenan that was seen in their experiment was that the 2.5% dose of carrageenan produced a slight increase in the size of the foci. Although stated to be statistically significant by the authors, there is great variability in the size of these foci in these animals and this could well have been within occurrence by chance. The fact that they were unable to reproduce their results in a follow-up experiment utilizing 2.0% and 2.5% carrageenan in jelly after AOM suggests that there actually was no effect in the first experiment. In addition, all experiments evaluating the carcinogenicity of carrageenan have not demonstrated any benign or malignant tumors with carrageenan itself. Based on the initiation promotion model, it would not be appropriate to refer to the effects seen in the experiment by Corpet et al. (1997b) as indicating promoting activity because:
The number of aberrant crypt foci (ACF) per colon actually decreased in rats given drinking water containing 0.25 and 2.5% carrageenan.
The apparent increase in multiplicity (crypts/ ACF) at 2.5% carrageenan of the drinking water was not statistically significant.
No effect was seen when the experiment was repeated in rats having their intestinal bacterial flora replaced with human microflora.
The response was not replicated by this same group of investigators in an experiment using 2% and 2.5% of carrageenan in drinking water when the rats were housed in isolators rather than cages.
A small number of rats were included in each group (10/group).
There was no evidence of the induction of preneoplastic lesions in the colon when carrageenan was administered by itself with out prior or concurrent administration of a known genotoxic intestinal carcinogen.
B. Aberrant Crypt Foci, Adenoma, Carcinoma Sequence
It is generally accepted that colon cancer in humans arises via two pathways (Owen and Kelly, 1996; Whiteley, 1999). The most common is the development of adenomatous polyps, whether tubular and/or villous, which then progress to carcinoma. Usually, high grade dysplasia occurs in the polyp before the development of invasiveness into the underlying submucosa and beyond. Considerable support for this pathway comes from numerous animal and human studies (Thorup, 1997; Fenoglio-Preiser and Noffsinger, 1999), including recent reports of surveillance programs using colonoscopy with polypectomies resulting in a marked reduction in the development of carcinomas.
However, there is a second pathway for the development of adenocarcinoma of the colon, involving nonpolypoid precursor lesions (Fenoglio-Preiser and Noffsinger, 1999). These have been referred to by a variety of names, but generally are included under the generic term of nonpolypoid dysplastic lesions. These are most notably identified in patients with the two Lynch syndromes in which carcinoma arises without the prior appearance of polyps.
As indicated above, it is essential that other types of polyps in the colon be identified and distinguished from adenomatous polyps, because other types do not have an increased propensity for the development of carcinoma. These include not only the hyperplastic polyps described above, but others, including hamartomatous polyps
(Peutz-Jeghers syndrome), inflammatory (juvenile) polyps, and a variety of nonepithelial polyps (Owen and Kelly, 1996).
Inasmuch as polyps are relatively large entities by the time they are detectable by colonoscopy in humans or grossly in animal experiments, it was long surmised that a precursor lesion must occur prior to the formation of the polyps. During the last decade, considerable evidence in rat and mouse models have identified aberrant crypt foci (ACF) as the precursor lesion to adenomatous polyps (Augenlicht et al., 1996; Fenoglio and Lane, 1974; Fenoglio et al., 1977; Fenoglio Preiser and Noffsinger, 1999; Thorup, 1997; Thorup et al., 1994; Kristiansen, 1996; Kristiansen et al., 1995; Kendall et al, 1992; McLellan and Bird, 1988; Takayama et al., 1998; Whiteley, 1999). These can be identified largely by staining of the colonic mucosal surface with methylene blue that accentuates the shape of the colonic crypt. Aberrant crypt foci have abnormalities in size and shape of the opening to the crypts that are readily visible by dissecting microscopy in animal experiments (Thorup, 1997), or by magnifying colonoscopy in humans (Yokota et al., 1997; DiGregorio et al., 1997). It does not appear that aberrant crypt foci are precursor lesions to the nonpolypoid pathway to the development of adenocarcinoma of the colon.
The glands within aberrant crypt foci show morphologic changes that are suggestive of adenomatous changes, with a decrease in goblet cells, increased proliferation of the crypt cells (Roncucci et al., 1993; Otori et al., 1995; Thorup, 1997), and molecular changes that are similar to some of those identified in adenomas, such as mutations in K-ras oncogene (Augenlicht et al., 1996; Fenoglio-Preiser and Noffsinger, 1999; Heinen et al., 1996; Losi et al., 1996; Nucci et al., 1997; Siu et al., 1999). Based on morphology, however, there are varying degrees of changes in these foci, with those foci containing glands most resembling adenomatous changes occasionally being classified as “dysplastic” (Caderni et al., 1991). Nevertheless, it remains unclear which foci represent glands that will evolve to adenomas or carcinomas because most foci do not appear to progress (Pereira and Khoury, 1991; Pretlow, 1995; Thorup, 1997; Thorup et al., 1994; Kristiansen et al., 1995; Whiteley, 1999).
In experimental animals and in humans, carcinogenesis in several organs involves a sequence of microscopic changes prior to the appearance of grossly detectable lesions. The earlier in the sequence one can identify changes, the less frequent the lesion is likely to progress into a fully developed carcinoma. For example, in the cervix the progression is from low-grade dysplasia to high grade dysplasia, ultimately with the development of carcinoma in situ and then invasive carcinoma. In the 1950s and 1960s, patients with these types of lesions were followed (Friedell, 1976; Friedell et al., 1960). It was demonstrated that not even all of the carcinomas in situ cases progressed to invasive carcinoma; less than half progressed within a 10-year span of time. Furthermore, significantly less than half of high-grade dysplastic lesions progressed to carcinoma in situ, and an even smaller percentage of low grade dysplasias progressed to high grade dysplasias and ultimately carcinomas. It appears that these early stage lesions are frequently reversible or do not progress. Similarly, most adenomatous polyps of the colon do not progress to invasive carcinoma in humans, although the exact percentage is not known (Whiteley, 1999; Fenoglio-Preiser and Noffsinger, 1999). Similarly, only a small percentage of aberrant crypt foci progress to adenomatous polyps (Thorup, 1997; Thorup et al., 1994; Kristiansen et al., 1995). In animal models and in humans, there is significant evidence that the adenomatous polyp pathway to adenocarcinoma of the colon begins with the development of aberrant crypt foci. However, present there are at no predictive morphologic, immunologic, or molecular markers to indicate how many or which ones of the ACF will progress to adenomas or carcinomas (Fenoglio Preiser and Noffsinger, 1999; Thorup, 1997).
Some authors have tried to make estimates ofwhat percentage of aberrant crypt foci in experimental models will develop into adenomas and carcinomas. As expected, the results have been highly variable, making predictive statements about this progression highly tenuous (Magnuson and Bird, 1993; Magnuson et al., 1993; Whiteley, 1999). In summary, aberrant crypt foci are a precursor lesion to the development of adenomas and carcinomas of the intestine, but the progression from foci to adenomas is infrequent and the foci appear to be potentially reversible.
C. Dietary Fiber and Colon Carcinogenesis
In 1969, Dr. Denis Burkitt (1969) postulated that high incidences of colon cancer in Western civilization were related to the low amounts of fiber in the diets of individuals in developed societies. This hypothesis has remained controversial ever since. There is no question that diet greatly influences the incidence of colorectal cancer, but which component or components are re- sponsible remain unclear (Giovannucci et al., 1992; Harris and Ferguson, 1993; Klurfeld, 1992; Winawer and Shike, 1992) . Epidemiological studies strongly suggest that ingestion of vegetables and fruits decrease risk of cancer, including colon cancer, whereas ingestion of meats generally increase the risks of colorectal cancer. The difficulty in dissecting out the critical components of diet are that they frequently occur together, such that ingestion of high fat is usually related to an excess of calories and low fiber in the diet (Trock et al., 1990; Lubin et al., 1997; Holt et al., 1998; Ahnen and Byers, 1998). Possibilities include not only dietary fiber, but dietary levels of fat (Shivapurkar et al., 1992), specific types of fat, total caloric intake, specific minerals such as calcium (Nelson,1987), or other constituents of plants such as polyphenols (McSherry et al., 1989; Shannon et al., 1996). Complicating these relationships even further are the complex nature of the components. Thus, the phrase “dietary fiber” actually represents a complex assortment of widely differing chemicals with significantly different influences on the biology of the intestinal tract. Foods with significant “fiber” content, such as grains, vegetables, and fruits, have numerous other substances that are known to affect the carcinogenic process, such as antioxidants, alkaloids, and polyphenols.
Dietary fiber is actually comprised of numerous different substances with different chemical- physical and biological properties. In general, they can be classified into starch, other soluble polysac- charides, and insoluble polysaccharides (Potter, 1999; Harris and Ferguson, 1999). However, the latter two categories contain a diverse number of substances, again with widely varying effects. Soluble fibers can be derived from microorganisms (xanthan gum, and gellan gum), seaweed (agar and carrageenan), exudate gums (gum arabic), or seeds (guar gum). Unfortunately, many investigators, particularly in epidemiological studies, have not distinguished between these different types of fibers, although they have widely differing effects on the gastrointestinal tract.
Given these wide varieties of substances classified as dietary fibers, it is not surprising that varying effects have been observed in different experiments and in epidemiological studies of the effects of dietary fiber on carcinogenesis (Zhang et al., 1992; Zoran et al., 1997; Corpet et al., 1997a; Potter, 1999, Harris and Ferguson, 1999). In general, experiments have suggested that most dietary fibers have a protective effect on colorectal carcinogenesis, both in animal experiments and in human epidemiological evaluations, but some studies have shown either no effect or actually a slight increase in carcinogenesis. A recent study (Fuchs et al., 1999) involving nearly 90,000 women illustrates many of the difficulties in trying to evaluate the effect of dietary fibers on colorectal carcinogenesis. In that study, there was no effect, either increase or decrease, of dietary fiber on colorectal carcinogenesis. However, al- though some attempt was made to distinguish between the different types of fiber, this was not entirely possible. Also, there was wide variation in fiber ingestion during the course (several years) of this prospective cohort study. It was also difficult to distinguish between the effects of fiber from caloric intake or dietary fat, as well as minerals and other plant substances.
Specific epidemiologic evaluation of a single dietary fiber, such as carrageenan, is not possible, and furthermore, separation of effects from dietary fat or total excess calories is not possible. Nevertheless, it appears from the literature that dietary fibers might protect against the development of colon carcinogenesis, if they have any effect on the process. Two more recent large epidemiologic studies cast further doubt on any relationship of fiber to colon carcinogenesis in humans (Schatzkin et al., 2000; Alberts et al., 2000).
V. SUMMARY AND CONCLUSIONS
Carrageenan and PES are members of a class of hydrocolloids widely used as ingredients in foods because of their excellent gelling properties and safe toxicological profile. Evaluations for carcinogenic activity in longterm bioassays have been negative in rats and hamsters, and less adequate studies have also been negative in mice. In addition, a longterm bioassay, 7.5 years, in monkeys was negative for tumorgenicity. All of these studies were at doses significantly higher than the maximally anticipated levels of ingestion by humans. At these high levels, in all species there is a tendency for production of watery stools and diarrhea. PES, a semirefined form of carrageenan, has been evaluated only to a limited extent in various assays, but the results have been similar to those observed with carrageenan.
Carrageenan is a large molecular weight polysaccharide that is not degraded in the gastrointestinal tract and is essentially not absorbed. PES contains, in addition to carrageenan, a small amount (< 15%) of cellulose that is also not de- graded in the gastrointestinal tract. Toxicological effects in oral studies are limited to the gastrointes- tinal tract, particularly the large intestine. In addition to the formation of watery stools and diarrhea in rodents administered high doses of carrageenan, there is enlargement of the cecum, which is particularly prominent in rats. Cecal enlargement is a common manifestation of alterations in intestinal absorption of water or as a manifestation of alterations in microflora secondary to high antibiotic consumption in non-primates. Alterations in water metabolism can be produced by either nondigestible polysaccharides, such as carrageenan, or by a variety of other substances that have a similar influence, such as saccharin. Cecal enlargement in non-primates is associated with hypertrophy and hyperplasia of the mucosal epithelium, with proliferation being largely restricted to cells at the mid to upper portions of the crypts and in fully differentiated cells. This effect does not appear to impact the proliferation of the stem cells at the base of the crypts. Influences on the intestine of high doses of carrageenan are similar to those seen with a variety of other fibers administered at high doses, such as oat bran, wheat bran, pectin, guar gum, or carboxy methylcellu lose (Elsenhaus et al., 1981; Mallett et al., 1984, 1985). The intestinal effects can be influenced considerably by diet composition, including total content of fiber, fat, and anions and cations The cecal effects vary considerably among animal species are greatly affected by diet and are difficult to extrapolate to humans because humans do not have a similar response in the cecum to these treatments. All of these alterations in intestinal structure and function in rodents are limited to administration of high doses well in excess of those ingested by humans. Lower doses do not produce any toxicological effects.
In addition to not being carcinogenic in animal bioassays, carrageenan and PES are also negative in a variety of in vitro genotoxicity tests and carrag- eenan is also negative in in vivo genotoxicity screens. This is to be expected because its chemical structure is that of a polyanion, which is not capable of chemically reacting with DNA. The interaction of chemicals with DNA requires a reactive cationic structure or a free radical. Thus, carrageenan is not capable of chemically reacting with DNA, and therefore is not anticipated to be mutagenic. This strongly supports its noncarcinogenicity in animal bioassays.
Recently, there has arisen some concern that carrageenan might have promoting activity in colon carcinogenesis (JECFA,1999). This is largely based on the results of experiments by Watanabe et al. (1978), Arakawa et al. (1986), and Corpet and his colleagues (Corpet et al., 1997b; Taché et al., 2000). The studies by Watanabe et al. (1978) and Arakawa et al. (1986) involve coadministration of carrageenan with a known colon carcinogen, either AOM, DMH, or MNU, and thus do not represent an evaluation for promoting activity. The studies by Watanabe et al. (1978) and Arakawa et al. (1986) had numerous flaws, making them unacceptable for further consideration regarding the safety of carrageenan in humans. The doses they used were up to 15% of the diet and were in excess of those accepted (FDA, 1982; Grice, 1984; OECD, 1981; 1998) as maximum for a chronic bioassay (5%). The MTD was exceeded, nutritional deficiencies were present in the diets, the high doses distorted the nutritional composition compared to the control diet, and cecal enlargement and watery stools were produced. These confounding factors could readily alter toxicokinetics and metabolism of the coadministered carcinogen.
The studies by Corpet and his colleagues (Corpet et al., 1997b; Taché et al., 2000) involved three experiments. In the first of these experiments, carrageenan was administered to rats at a dose of 0.25% and 2.5% of the diet. Aberrant crypt foci served as a surrogate marker for the later development of adenoma and/or carcinomas in these studies. At both doses, there was a significant decrease in the number of aberrant crypt foci produced. However, there was a marginally statistically significant increase in the size of the foci. Again, this might have occurred secondary to the alterations on colonic function that are seen following high doses of carrageenan, but also might not be an actual effect because it was of marginal statistical significance, and this response (number and size of ACF) is highly variable in the rat colon. Supporting the likelihood that this was a statistical aberration and not a biologically significant response is the fact that these authors were unable to reproduce their finding when they repeated the experiment at doses of 2.0 and 2.5% in the diet. When they repeated the experiment, the number of foci and their size in rats administered carrageenan after AOM was similar to that seen in controls. In addition, no effect was seen when carrageenan was administered at a dose of 2.5% to rats that had their rat microflora replaced with human microflora. Again, even if there was a slight effect, which seems unlikely, it is difficult to evaluate what this means with respect to carcinogenesis. It does not represent promotion because the number of lesions was actually decreased. The size of the lesions could easily have been altered by the fact that the cecum was enlarged.
In addition, there has been some concern that carrageenan leads to an increase in cecal epithelial cell proliferation, and that this might contribute to a carcinogenic effect. This seems highly unlikely because the proliferation that has been identified does not appear to occur in the critical target cells for colon carcinogenesis, the crypt stem cells. Rather, the proliferation appears to occur in either the upper levels of the epithelium, representing differentiating cells with an increase in mucin-producing goblet cells, or possibly even an increase in the proliferation of the numerous mononuclear cells that are present in the lamina propria and submucosa. When morphologic methods were utilized to assess cell proliferation, such as 3H-thymidine incorporation and autoradiography, no increase in proliferation was seen or the proliferation was in the upper portion of the crypts with no increase in the crypt stem cells at the base of the crypts. When biochemical methods are utilized, such as increased thymidine kinase enzyme activity, utilizing a homogenate of the entire mucosa, it is impossible to interpret what cell types are actually proliferating. It is highly unlikely, given the extremely small percentage of total cells present in the assay following scraping of the colonic mucosa, that the few cells present that are colonic epithelial crypt stem cells could be detected with this assay.
In summary, carrageenan has not been shown to be carcinogenic in a variety of species, includ- ing rats, hamsters, monkeys, and mice. It is not genotoxic and its chemical structure is not anticipated to allow for reactivity with DNA. There is no evidence that supports an increase in proliferation of the target cells in the intestine that are critical for carcinogenesis, the crypt stem cells, with no indication of promoting activity of carcinogenesis. Experiments with PES are more limited, but from its chemical composition there is no reason to expect it to behave any differently than carrageenan.
In 1983, the International Agency for Re- search on Cancer reviewed the data and concluded that the available data did not provide evidence that carrageenan is carcinogenic to experimental animals. Subsequent data provide no evidence to suggest a change in this conclusion. Previous reviews by JECFA have concluded with a group acceptable daily intake (ADI) “not specified” for carrageenan and PES. Carrageenan is not carcinogenic in experimental animals, is not genotoxic, and does not have tumor-promoting activity for colon carcinogenesis in rats. PES is similar to carrageenan to the extent it has been investigated and it is expected to behave similarly to carrageenan.
The information contained in this article was submitted to the Joint FAO/WHO Expert Committee on Food Additives (JECFA) for review at their 57th meeting in June 2001. Their review resulted in the assignment of an ADI of “not specified”.